Bacteria can help us cure autoimmune disease
The human immune system is very powerful, and if it is directed towards ourselves, it can lead to the very serious so-called autoimmune diseases
Most of the time our immune system does its job efficiently without us noticing. It clears our bodies from potentially harmful bacteria, fungi and viruses, but it is also involved in removing or destroying our own cells if given the signal that a particular cell is damaged or diseased (i.e. viral infections or transformation into cancer cells).
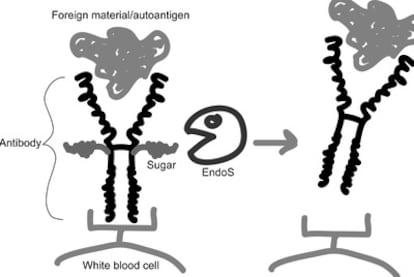
The human immune system is very powerful, and if it?for genetic, environmental, or unknown reasons?is directed towards ourselves, it can lead to the very serious so-called autoimmune diseases. An autoimmune disease can affect specific organ systems, such as joints (rheumatoid arthritis) or the central nervous system (multiple sclerosis), or more or less the whole body (systemic lupus erythematosus or SLE). A central component in many autoimmune diseases is autoantibodies, which are antibodies directed towards our own tissues (autoantigen) instead of foreign material. The binding of autoantibodies to our tissues leads to the activation of white bloods cells and other accessory components of our immune system, resulting in a massive inflammation and destruction of cells and tissues (see figure).
Current therapies of antibody-mediated autoimmune disease focus on reducing the amount of autoantibodies by using general immunosuppressants such as corticosteroids or chemotherapy, by physical removal of antibodies by a process called plasmapherisis, or by using other antibodies towards the antibody producing white blood cells.
In the hunt for new treatments for antibody-mediated autoimmune disease, we turned to the real experts on modulation of the human immune system - disease-causing bacteria. Our rationale is that bacteria have co-evolved with their human and animal hosts for a very long time, and bacteria would have been extinct if they would have not found ways to circumvent the host's immune defense. We focused on the bacteria group A streptococci (GAS, i.e. "strep throat" bacteria), which have multiple ways of avoiding the immune system.
We were able to identify a novel GAS immunomodulating factor, the enzyme EndoS. EndoS removes sugars from human antibodies of IgG type and thereby blocks activation of white blood cells and accessory factors (see figure). This is of obvious benefit for the bacteria?during infection, the bacteria can avoid being recognized by antibodies and killed by white blood cells. But when EndoS is taken out of its bacterial context in a purified form, we got the idea that it instead could be used block the harmful effects of autoantibodies.
In order to test this, we had to turn to animal models of autoimmune disease. We could clearly show that EndoS very efficiently removes the sugars of all IgG antibodies in rabbits without causing any side effects, that EndoS blocks the development of antibody-mediated arthritis in mice, that EndoS cures mice from a lethal bleeding disorder caused by autoantibodies against platelets, and that EndoS prolongs the life of mice that spontaneously develop the severe autoimmune disease SLE.
Even though there is still a long winding road before EndoS can be tested as therapy against autoimmune diseases in humans, it represents one of the most promising and unique approaches to treat autoimmune disease that has been presented for many years. The story of EndoS also stresses the importance of studies on host-parasite interactions in order to deepen the understanding of how the immune system works as well as how it can be manipulated to cure disease.
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.




























































