New frontiers for solving the proteome puzzle
The term "proteome" refers to the structure, function and interactions of peptides and polypeptides (i.e., proteins) under defined conditions and in a defined region (ranging from sub-cellular structures to the whole body) of a living organism. Peptides and proteins are key pieces in the biological puzzle of interwoven molecular networks involved in the human body and contain many of the answers to the mysteries of life. The basic building blocks of these molecules are the twenty standard alpha-amino acids. These blocks are arranged in sequences that are specified as a part of the genome - the whole genetic material of an organism (the DNA in most cases).
Genomics and proteomics are the study of the genome and the proteome, respectively. Miniaturized high-performance separation techniques - such as capillary electrophoresis (CE) - may play a crucial role in both research fields because the total volume of sample available for analysis is typically extremely limited. This leading-edge analytical technology consumes only minute amounts of samples, reagents and solvents, allowing separation of a high number of components in extremely short times and at reasonable cost. Furthermore, some instrumental configurations also enable fully automated running of a massive number of samples in parallel.
A number of challenges remain to be solved with regard to sensitivity measurements, especially for proteomics research due to the low concentration of some of the most interesting target peptides and proteins. The high-resolution power of CE permits precise characterization of molecules from complex samples. During CE analysis, the molecules move according to their charge-to-size ratios under the influence of a high electric field in a conductive liquid which fills a 50-70 cm glass-like column with an internal diameter of only a few times larger than the smallest blood vessels of the human body. However, the limited volume of sample needed for optimal separation makes the measurement of the separated compounds difficult, resulting in poor concentration limits of detection. This major drawback has been overcome in genomics by amplifying the fragments of DNA to generate millions or billions of copies using the polymerase chain reaction (PCR). In fact, CE was largely responsible for the completion of the Human Genome Project in record time.
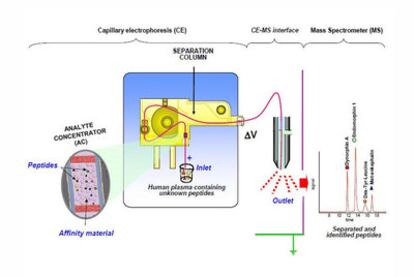
Unlike DNA sequencing, there is no amplification method available for peptides and proteins. Furthermore, the analytical challenge to decipher the proteome is even greater, because of the increased number and complexity of the targets and the limited quantity of most of these targets in different biological samples. Our research in the Bioanalysis group of the Department of Analytical Chemistry of the University of Barcelona focuses on the development of novel strategies for handling peptides and proteins on a microscale level in highly diluted samples using high-performance separation techniques. We concentrate in particular on CE coupled to a mass spectrometry detector, which allows identification of the separated compounds after measuring their molecular mass. In order to improve sensitivity for low abundance peptides and proteins, we are developing an original modification of CE, which requires laboratory preparation of a 0.8 cm long microdevice called an "analyte concentrator (AC)" (Figure 1).
The AC is placed near the inlet of the separation column and works like an efficient magnet pulling a needle in a haystack. The AC contains a solid material with high affinity (e.g., specific antibodies) for the peptides or proteins of interest. The affinity material selectively retains the target molecules when the sample is loaded, enabling large volumes of sample to be introduced for the separation (more than 1000 times the volume of sample analyzed in conventional CE, which is around 0.00005 millilitres). The captured molecules are extracted in a smaller volume of an appropriate solution. As well as resulting in an increased concentration, this results in the removal of interfering components with minimum sample handling, allowing more sensitive measurements to be made. The higher the binding capacity of the affinity material, the better the overall improvement in sensitivity. We are now able to detect and identify peptides in human plasma samples at concentrations some 10 000 times lower than those typically detected by CE. We are currently working on the development of high capacity affinity materials to achieve a 100 000 fold improvement. This will enable the ready identification of peptides and proteins in low-concentration proteome fractions. We are also carrying out research to understand and optimize all the operational parameters involved in the use of the AC technology in order to take advantage of its great potential in routine analysis for discovery of biomarkers of fibromyalgia, chronic pain syndrome and Huntington's disease.
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.