Intracellular calpain activity: a pointer to disorders of the ageing brain?
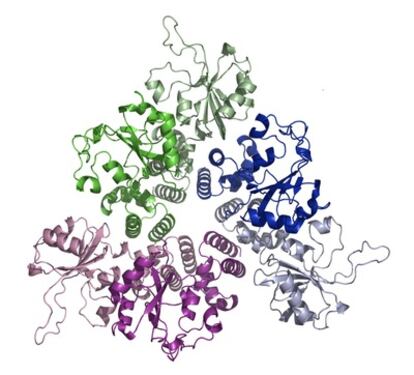
If a protein breaks up, it can no longer carry out its specific function. Calpains, therefore, influence the biological function of the substrates?proteins, in this case. The exact effect of this cleavage depends a great deal on the role assigned to the intact protein in a normal cell but it is known that the splitting is associated with the development of such diseases as Alzheimer's, Huntington's, and Parkinson's as well as non-insulin-dependent diabetes. Although calpains are apparently involved in many physiological and pathological changes, their functions are far from clear as yet.
Different methods are used to study the processes in which calpains are involved. One method stimulates excess production of calpains by manipulating the appropriate gene or genes controlling the expression of the calpains whereas another removes them altogether: we can then observe what happens when there is excess of calpain or when it is missing altogether. Calpain function can also be inhibited by small molecules - the inhibitors - which can enter a cell through its membrane, and we can compare the cells so treated with normal, untreated cells to study the role of the calpain that was thus inhibited. Unfortunately, this method is not precise because the known inhibitors are not selective enough?they inhibit other enzymes too, which means that we cannot say with confidence that the change we observed was only due to the particular calpain. Manipulation of specific genes is a precise method but the technique needs a well-equipped laboratory and is not simple.
Compounds that are highly active biologically and are selective in their effects often cannot cross the cell membrane and thus cannot act on their target within the cells. The solution is therefore to attach such compounds to peptides - cell-penetrating peptides - that can cross the cell membrane to enter a cell with their cargo.
In my research, I prepare and study bioconjugates, which are molecules that contain two or more biologically active compounds and a cell-penetrating peptide. In our research on calpains, the peptides come from calpastatin, a protein that can inhibit calpains selectively. Surprisingly, although calpastatin inhibits calpains, peptides derived from calpastatin stimulate the activity of calpain by binding to it selectively. Because these long peptides cannot enter cells on their own, we attached them to (or, to use the technical term, conjugate them with) a peptide that can. When cells are treated with such conjugates, the conjugates can enter the cells and increase enzyme activity inside the cells. The conjugates thus offer a simple and selective method to study the function of calpains: we measure a cell function in normal cells, incubate the cells in a solution of conjugates, and measure the same function once again. The difference between the two sets of measurements can tell us something about the role of the calpain.
Now what we need is a substrate on which the calpain can act to produce a difference large enough to be easily measurable. This substrate should either be a normal constituent of a cell - a well-known protein substrate, for example - or one that can be easily transported into a cell?a peptide substrate. To find such a peptide substrate, we examined many protein substrates and studied the points at which they had been cleaved by the calpain into peptides that differed in their constituent amino acids. Based on the results, we selected a peptide composed of 11 amino acids as a suitable substrate. Calpain activity is measured in terms of how fast it can cleave a substrate, which means we need a product of such cleavage that can be readily measured. One way is to use a fluorescent dye and a non-fluorescent dye and attach them to the substrate peptide. The fluorescent dye emits light after excitation, light that is absorbed by the non-fluorescent dye. Thus the fluorescent signal - the emitted light - cannot be detected if the light-absorbing molecule and the fluorescent dye are too close to each other whereas if the two are separated in space, the fluorescence can be detected easily. And it is the enzyme that cleaves or splits the substrate so that the dyes drift away from each other. The more intense the emitted light after excitation, the greater the amount of the cleaved peptides. Although the 11-amino-acid-long peptide that we selected cannot enter a cell on its own, we synthesized its conjugate with a cell-penetrating peptide that can, and were thus able to measure calpain activity within the cells.
The outcome of our research is a set of tools to modify or simply to measure intracellular calpain activity. Using such measurements we can obtain more data about known functions of calpain or even identify new functions.
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.